TT (Tetanus toxoid)
Z23.5
Protects against tetanus (neonatal and after wounds)
- TT, IM, 0.5 mL into arm
- Storage:
- Fridge: middle shelf at 2–8℃.
- Easily damaged by freezing.
- Keep opened vials for next session if kept at correct temperature and not contaminated.
- Discard after 30 days.
- Record date of reconstitution.
- Contraindications:
- Previous anaphylaxis.
- Storage:
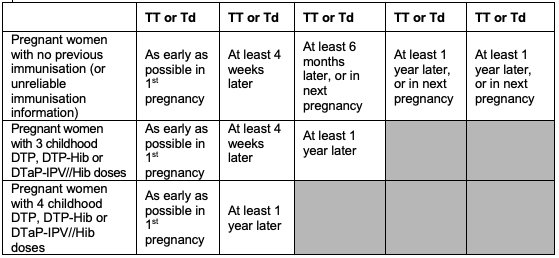
Pregnant women
All pregnant women should routinely receive tetanus toxoid.
Trauma
- Give booster dose of TT/Td after each trauma episode (unless given in previous 5 years).
Human Papilloma Virus (HPV) Vaccine
Z25.8
Protects against infection with HPV serotypes 16 and 18.
Persistent HPV infection is associated with the development of a number of reproductive tract cancers, especially cancer of the cervix.
Two dose schedule (6 months apart) currently offered as part of the Integrated School Health programme to Grade 4 girls (≥ 9 years of age) in public schools.
- HPV, IM, 0.5 mL
- Administered into the deltoid of the non-dominant arm.
- Storage:
- Fridge: middle shelf at 2–8℃.
- Easily damaged by freezing – do not freeze and discard any vaccine which has been frozen.
- Store in original package and protect from light.
- Use immediately once withdrawn into a syringe.
- Contraindications:
- Previous anaphylaxis.
- Febrile illness (≥ 38.5℃).
- Should not be administered to girls/women who are known to be pregnant.
- Adverse events:
- Injection site pain and swelling in the arm are common.
- Itching, rash, redness and urticaria may also occur.
- Nausea, diarrhoea, abdominal pain, headache, myalgia, fever (38℃) are not uncommon.
- Syncope, dizziness, lymphadenopathy, and anaphylaxis have been reported.
Hepatitis B
Z24.6
All personnel working in a health care facility (including support staff)
- Hepatitis B, vaccine, IM, 3 adult doses of 1 mL.
- first dose administered immediately;
- second dose 1 month after the first dose;
- third dose 6 months after the first dose.
Perinatal transmission
Babies born to mothers with acute hepatitis B infection at the time of delivery or to mothers who are HBsAg-positive or HBeAg-positive, see: Perinatal transmission of hepatitis B.
Influenza vaccine
Z25.1
- Influenza vaccine, IM, 0.5 mL.
- Contraindication: severe egg allergy, < 6 months of age.
- All women who are pregnant at the time of the annual immunisation campaign should be immunised.
- People with the following risk factors may be offered immunisation during the annual campaign:
- HIV infection.
- Chronic cardiac or pulmonary conditions.
- Age > 65 years.
- Healthcare workers are not routinely offered immunisation during the annual campaign. Although it is recommended that healthcare workers are vaccinated against influenza, they will not be provided with publically funded vaccines unless they fall within any of the designated high risk groups.
NOTE: Prioritisation strategies may vary in a pandemic.
Recommended dosage of influenza vaccine for patients of different age groups:
| Age group | Dose | Number of doses |
|---|---|---|
| Adults and children ≥9 years | 0.5 mL, IM | Single dose. |
| Children:>3 to <9 years | 0.5 mL, IM |
2 doses ≥ 4 weeks apart during first year of immunisation, thereafter one dose per annum. |
| Children: >6 months to <3 years | 0.25 mL, IM |
2 doses ≥ 4 weeks apart during first year of immunisation, thereafter one dose per annum. |