B20-24
DESCRIPTION
An infant or child in whom HIV infection has been confirmed with 2 appropriate tests.
For confirmation of HIV infection see Human immunodeficiency virus infections .
GENERAL AND SUPPORTIVE MEASURES
Counselling is a vital part of the successful care of children with HIV infection and their families. Specific matters requiring attention are:
- The implications of the disease to the family.
- Implications of treatment, non-adherence and understanding of the condition and its care.
- The disclosure process within the family and extended family/friends should be encouraged. Help from the family/friends is often useful.
- Disclosure to the child of appropriate age and maturity.
Treatment of mothers, caregivers and other family members:
- Always ask about the caregiver’s health, and the health of other members of the family.
- Ensure that mothers and other family members have timeous access to medical care including cART.
- Encourage breastfeeding in all mothers with HIV infected children, with introduction of weaning foods from 6 months of age. Breastfeeding duration is recommended for 2 years or longer, as in HIV unexposed children.
- Always ask at every visit about TB contacts and TB symptoms in all children and their caregivers.
STANDARDISED NATIONAL MONITORING FOR INFANTS AND CHILDREN WITH HIV
| At initial diagnosis of HIV | Purpose |
|---|---|
| Verify HIV status. |
To ensure that national testing algorithm has been followed. |
|
Document weight, height, head circumference (HC if < 2 years of age) and development. |
To monitor growth and development. |
| Screen for TB symptoms. | To identify TB co-infection. |
| Do CD4 count. |
Children <5 years of age: Baseline. Do not wait for CD4 count to start ART. |
| Do CD4 count. |
Children ≥5 years of age: To determine eligibility for ART. |
| Hb or FBC if available. | To detect anaemia or neutropenia. |
| At Initiation of cART (baseline) | Purpose |
| Hb or FBC. | If less than 8g/dL, manage appropriately. |
| CD4 count (if not performed in last 6 months). | Baseline assessment. |
|
Cholesterol + triglyceride if starting PI-based regimen. |
Baseline assessment. |
|
ALT (if jaundiced or on TB treatment). |
To assess for liver dysfunction at baseline. |
| On cART | Purpose |
|
Height, weight, head circumference (HC if <2 years of age) and development. |
To monitor growth and development stages. Adjust dosing at each visit as necessary according to weight gain. |
|
Clinical assessment including drug-related adverse events. |
To monitor response to ART and exclude adverse effects. |
|
CD4 count: At 1 year on ART, and then every 12 months thereafter. |
To monitor response to ART and stop co- trimoxazole prophylaxis as indicated. |
|
Viral load (VL): At month 6, 1 year into ART, then every 12 months. |
To monitor viral suppression on ART. To identify treatment failure and identify adherence problems. |
|
Hb or FBC at month 1, 2, 3 and then annually if on AZT. |
To identify AZT-related anaemia. |
|
Cholesterol + triglyceride at 1 year of treatment and then every 12 months if on PI-based regimen. |
To monitor for PI-related metabolic side effects. |
MEDICINE TREATMENT
Co-trimoxazole prophylaxis
Indications:
- All HIV exposed infants starting from 4–6 weeks of age.
Discontinuation:
- If the child is shown to be uninfected and has not been breastfed for the last 6 weeks; or
- If HIV-infected, the immune system is fully reconstituted on cART and child > 1 year of age (i.e. child 1 to 5 years of age: CD4 >25%, or child > 5 years of age: CD4 > 350 cells/mm³ on 2 tests at least 3–6 months apart).
- Co-trimoxazole (sulfamethoxazole/trimethoprim), oral, once daily (everyday).
| Recommended daily dosage by weight band |
Dose sulfamethoxazole/ trimethoprim |
Suspension 200/40mg per 5mL |
Single strength tablet 400/80mg |
Doublestrength tablet 800/160mg |
|---|---|---|---|---|
| 3 to 4.9 kg | 100/20mg | 2.5 mL | ¼ tablet | - |
| 5 to 13.9 kg | 200/40mg | 5 mL | ½ tablet | - |
| 14 to 29.9 kg | 400/80mg | 10 mL | 1 tablet | ½ tablet |
| >30 kg | 800/160mg | - | 2 tablets | 1 tablet |
Immunisation, deworming and vitamin A program
Continue deworming and vitamin A programme as in the HIV-negative child.
Continue immunisation as in the HIV-negative child. See the Standard Treatment Guidelines and Essential Medicines List for Primary Health Care, Immunisation.
Nutritional support
Specific nutritional conditions should be treated appropriately.
Antiretroviral therapy (ART)
Initiation of cART in well uncomplicated infants shown to be PCR positive should be at PHC level – see national NIMART guidelines (IMCI) and Standard Treatment Guidelines and Essential Medicines List for Primary Health Care.
The preparation of the child and family to start cART is critical to the success of the treatment. Failure to achieve adherence may lead to resistance and adversely affect the prognosis of the child.
Eligibility criteria for antiretroviral therapy
- Confirmation of diagnosis of HIV infection irrespective of CD4 count or WHO clinical staging.
and - No medical contraindication (e.g. major organ dysfunction). If medical contraindications are present refer to hospital for rapid review and planning.
Children requiring fast track (i.e. to start cART within 7 days of being eligible with attention to social issues, counselling and adherence)
- Children <1 year of age.
- CD4 count <200 cells/mm³ or <15%.
- WHO Clinical stage IV.
- MDR or XDR-TB infection unless TB meningitis is diagnosed.
Social issues that must be addressed to ensure successful treatment
These are extremely important for success as they impact on adherence.
Social challenges should be overcome and not be barriers to care.
Disclosure to another adult living in the same house is encouraged so that there is someone else who can assist with the child’s treatment.
- Mandatory component: at least one identifiable caregiver able to supervise the child and/or administer medication. All efforts should be made to ensure that the social circumstances of vulnerable children, e.g. orphans, be addressed to facilitate treatment.
- Adherence:
- high levels of adherence (>95%) should be attained for adequate virological response and prevention of viral resistance. This can be achieved with regular education and support.
- All efforts to encourage this level of adherence should be made.
- Viral load measurements are useful for monitoring adherence.
- Sensitive, age-appropriate disclosure may facilitate adherence.
Requirements before cART is used
The child’s family (parents, caregivers) should understand:
- that antiretroviral therapy is lifelong;
- the prognosis of the condition (treated and untreated);
- adverse effects of the medicines, their mode of action, and the risk and implications of developing resistance, if incorrectly used;
- that all medications should be given as prescribed.
ART Regimens
- Are chosen according to age, weight, expected adverse effects, efficacy and prior antiretroviral exposure.
- Adjust the dosage of antiretroviral therapy according to weight during follow up visits. Assess weight gain and need for adjustment at each visit.
- Do not change regimens or move to 2ⁿᵈ line therapy without clear guidance from an experienced practitioner in child ARV medicine as unnecessary loss of effective regimens can shorten life expectancy. Adherence problems need to be addressed thoroughly before switching to a 2ⁿᵈ or 3ʳᵈ line regimen.
- Single drug substitution may only be made when drug-specific adverse effects are encountered, on condition that complete virological suppression is documented and the matter is discussed with an experienced practitioner in child ARV medicine first.
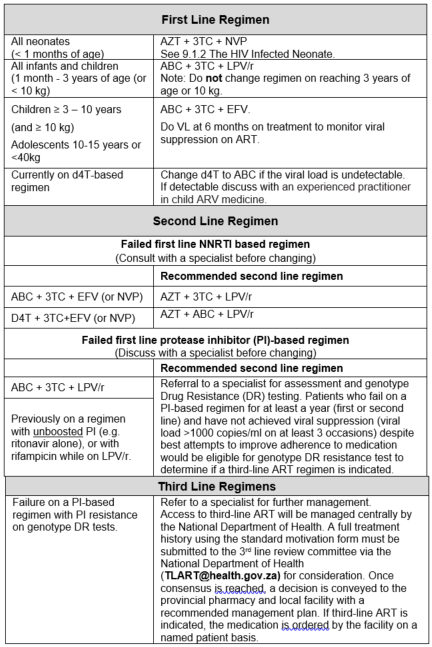
First Line Regimen for All neonates see The HIV infected neonate (less than 1 month of age)
General comments
Switch to tablets or capsules from syrups or solutions as soon as possible.
Use fixed dose combinations in preference to single agents. If available, use daily dose regimens.
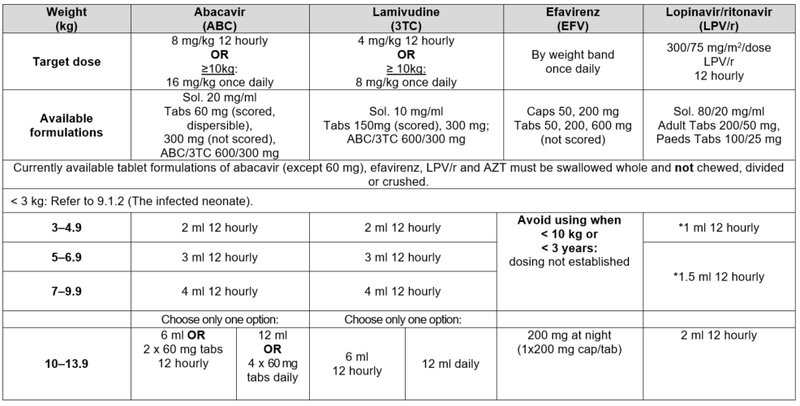
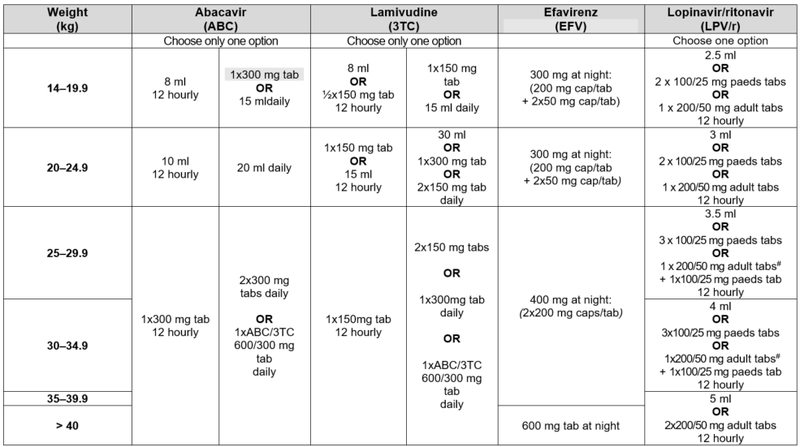
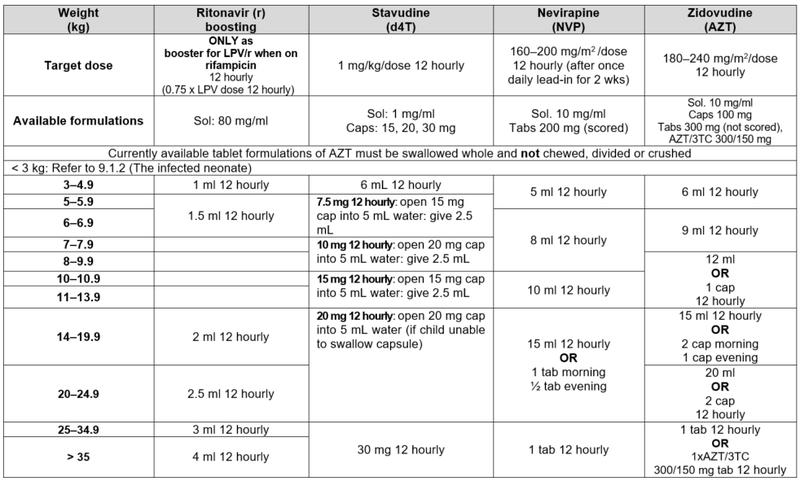
* Avoid LPV/r solution in any full term infant <14 days of age and any premature infant <14 days after their due date of delivery (40 weeks post conception) or obtain expert advice. #Children 25–34.9 kg may also be dosed with LPV/r 200/50 mg adult tabs: 2 tabs am; 1 tab pm.
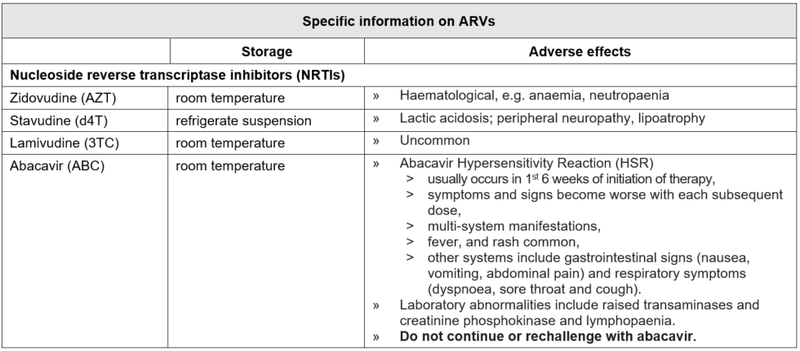
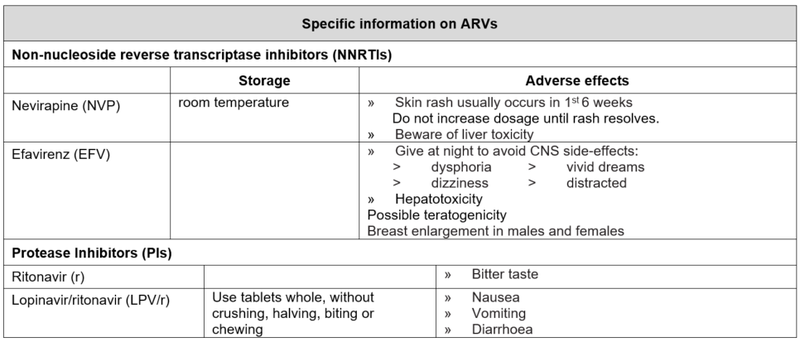
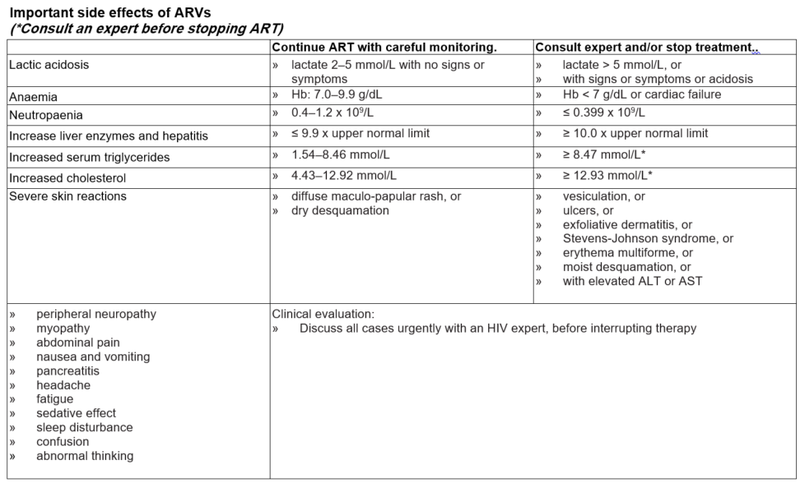
Criteria for changing therapy
Adverse effects
Children may occasionally need to change a medicine from the first line regimen to one from the second line regimen because of intolerance or a serious adverse reaction. There is no need to change an entire regimen for a single adverse drug reaction.
Note: a single drug substitution can only be made if the viral load is undetectable or if the change is made in the first six months of starting a regimen. The decision to swap must be made by a doctor with antiretroviral experience (this can be by telephonic consultation), as inappropriate choices of antiretrovirals may be ineffective or dangerous.
Treatment failure
The HIV viral load is the most sensitive method to detect failure of response to ART.
Virological failure can be defined as a measurable viral load despite optimal adherence and optimal dosage over a four-month period. Treatment failure is defined primarily by viral loads, as waiting for clinical or immunological failure enhances the chances of increasing viral resistance to other available anti-retroviral agents.
The most common cause of failure of first (and subsequent) line therapy is poor adherence. There is no point in changing to second line therapy before adherence has been addressed.
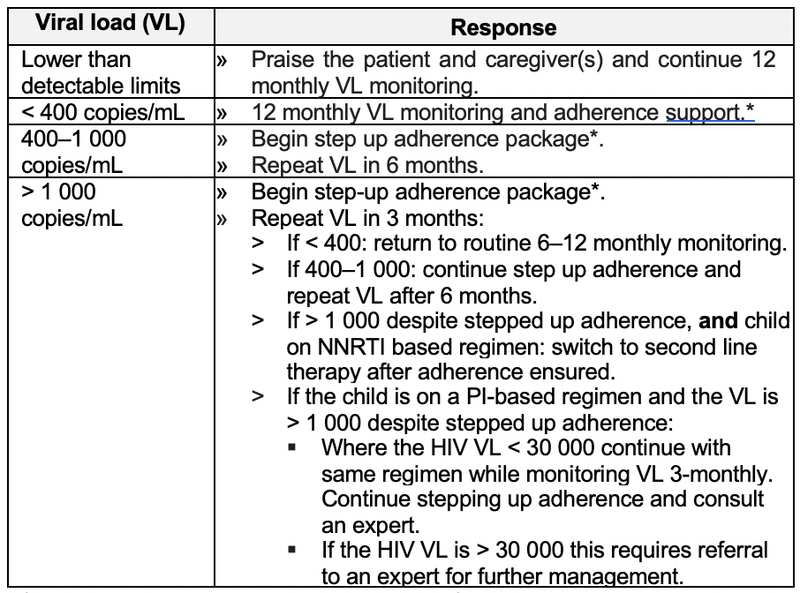
*For guidance on step-up adherence package refer to National adherence guidelines
REFERRAL
- Complicated or very ill children should be referred to a practitioner skilled in the care of such children.
- Attempts should be made to refer patients to accredited primary health care sites once stable on ART.