B20-24
DESCRIPTION
Adolescence encompasses the period of physical and psychological development from the onset of puberty to maturity. HIV in adolescents may be due to:
- Vertical infection in infancy that presents as long term non-progressors; or
- Sexually acquired HIV from unprotected intercourse.
Increasing numbers of perinatally infected infants are surviving to adolescence.
Adolescence is a high risk period for non-adherence to therapy. Mood disorders, denial, peer pressure, self-esteem and suicide risk are more common and patients may need to be referred for psychological support.
Education about sexual and reproductive health should be commenced early. Every encounter with the adolescent needs to be maximally utilised to discuss condom and contraception use to protect against unplanned pregnancies and STI transmission including HIV is essential. Schools should be taking an active role in this education. Sexually active youth need to be screened for STI symptoms and managed appropriately.
Consent
For testing, treatment and disclosure, the current acts and regulations should be followed.
Disclosure
All adolescents need to be aware of their HIV status. This should be handled sensitively. In addition, disclosure of diagnosis has ramifications for adherence. Disclosure should be planned with the caregiver and usually takes place over 2–3 visits. Disclosure should start in childhood using non-specific terms such as “germ” and “medicine”, building up to full disclosure around 10 years of age. Intervention by a social worker is useful where appropriate, although disclosure is often managed by skilled counsellors. Determine what the adolescent already knows and discuss with the caregiver about who should disclose and where.
Dosage of ARVs
In children over the age of 15 years and over 40 kg use adult dosage regimes – consult ART guideline.
Transition from Paediatric cART regimens to adolescent/adult regimens
- Adolescents with an undetectable VL (< 50 copies/mL) and no side effects on ABC + 3TC + EFV can remain on the same regimen until the patient becomes eligible for the TDF + FTC + EFV (FDC) at 15 years old and weighing ≥ 40kg.
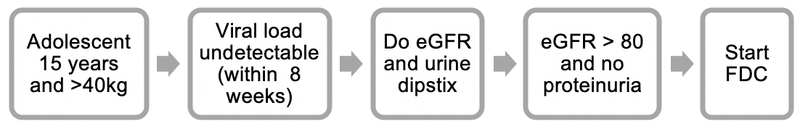
- When an adolescent with an undetectable viral load (taken within the last 8 weeks) reaches 15 years of age and is ≥ 40kg, a creatinine level, calculate the estimated glomerular filtration rate (eGFR) using a standard formula, and urine strip test should be performed.
- If the eGFR is > 80mL/min and no proteinuria on urine strip test, then the patient can be switched to the FDC (TDF + FTC + EFV).
- If the eGFR is < 80mL/min or > 1+ proteinuria on urine strip test, then refer to an expert for advice before switching.
Transition from child-adolescent regimen
If the HIV VL is between 50-1000 copies/mL consult an expert for advice.
If the HIV VL is > 1000 copies/mL, exclude non-adherence then treat as virological failure.
Contraception in HIV infected adolescents on cART
Hormonal contraceptives and IUCDs do not prevent sexually transmitted infections.
Additional use of condoms is required.
- Intra-uterine contraceptive device (IUCD): HIV is not a contraindication to IUCD use and may be used in adolescents on cART e.g. 380mm² copper – standard type.
- Progestogen-only subdermal implant contraceptive e.g. Levonorgestrel, 150mg, subdermal two-rod implant.
Note: Progestogen-only subdermal implant should NOT be used in patients on efavirenz. Additional non-hormonal contraception is required during and for up to 28 days after discontinuation of enzyme-inducing agents including rifampicin, efavirenz, and many anticonvulsants (e.g. carbamazepine, phenobarbital, phenytoin)
LoEII [6]
LoEII [7] - Injectable contraception: e.g. Medroxyprogesterone acetate (long-acting), IM, 150 mg, 12 weekly.
Note: It is unnecessary to shorten the dosage interval for women taking concomitant enzyme-inducing drugs, e.g. rifampicin, antiretrovirals and anticonvulsants. - Combined oral contraceptives (COCs) are indicated for motivated patients where adherence is more likely but are associated with drug-drug interactions.