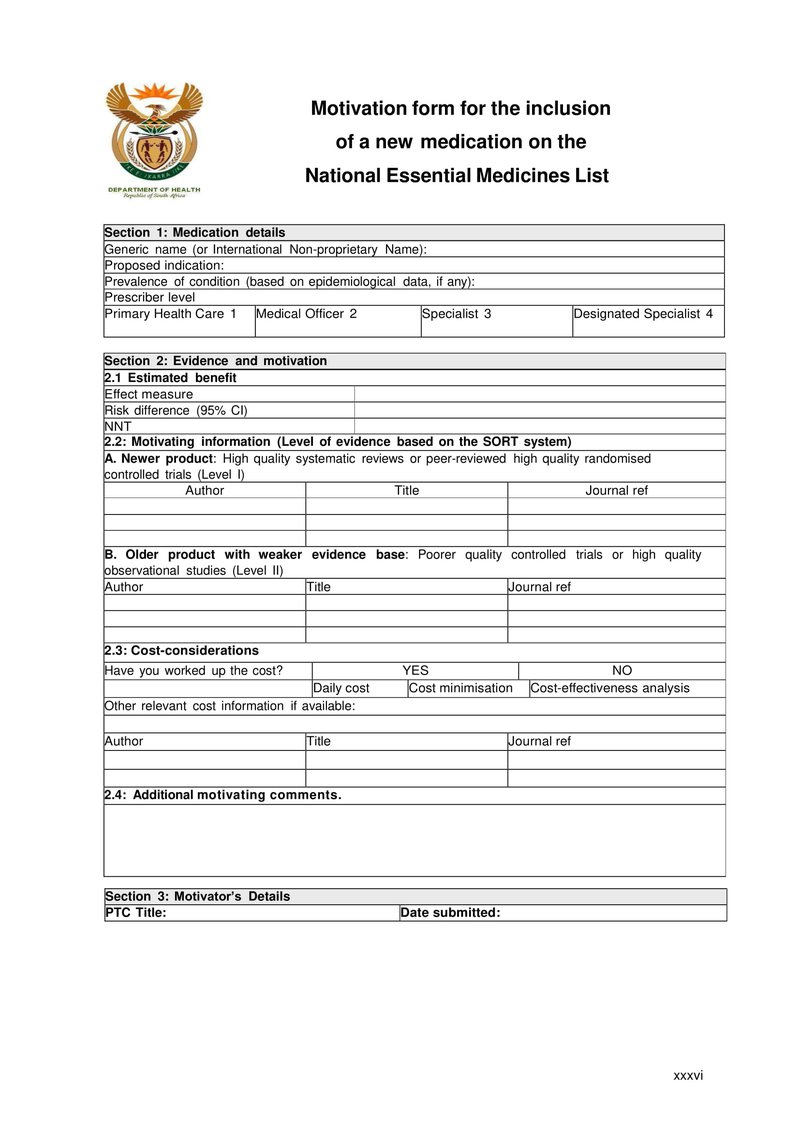
Section 1: Medication details
- Generic name
A fundamental principle of the Essential Drug Programme is that of generic prescribing. Most clinical trials are conducted using the generic name. - Proposed indication
There will usually be many registered indications for the medication. However, this section should be limited to the main indication, which is supported by the evidence provided in section 2. - Prevalence of the condition in South Africa
This information is not always readily available. However, it is an important consideration in the review of a proposed essential medicine. - Prescriber level
Here the proposed prescriber level should be included. If more than one level is proposed each relevant box should be ticked.
Section 2: Evidence and motivation
- Estimated benefit:
- Effect measure: this is the clinical outcome that was reported in the clinical trial such as BP, FEV, CD₄, VL etc.
- Risk benefit: this should be reported in the clinical trial and, in most cases, includes the 95% confidence level (95% CI). Absolute risk reduction, also termed risk difference, is the difference between the absolute risk of an event in the intervention group and the absolute risk in the control group.
- Number Needed to Treat (NNT): gives the number of patients who need to be treated for a certain period of time to prevent one event. It is the reciprocal of the absolute risk or can be calculated using the formula below.
Calculations
| Bad Outcome | Good Outcome | Total Patients | |
|---|---|---|---|
| Intervention group | a | c | a+c |
| Control group | b | d | b+d |
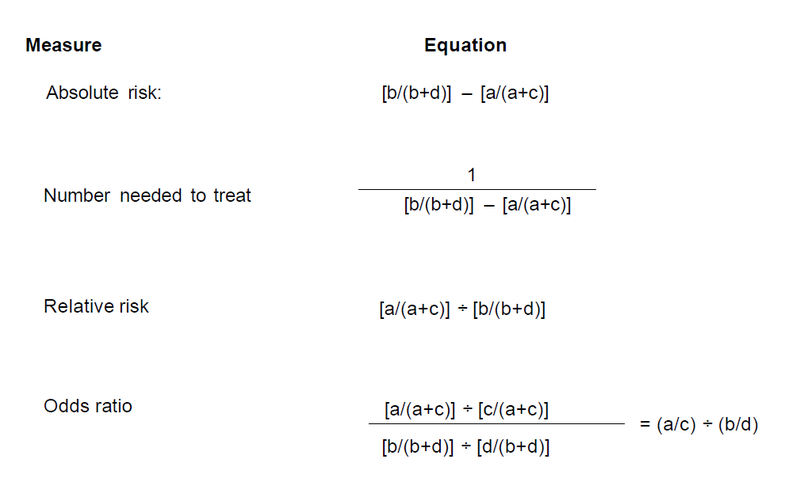
Reference - Aust Prescr 2008;31:12-16
- Motivating information , (Level of evidence based on the SORT system)
The National Essential Drug List Committee has endorsed the adoption of the SORT system for categorising levels of evidence. This system1 contains only three levels:
| Level I | Good quality evidence |
Systematic review of RCTs with consistent findings High quality individual RCT |
| Level II | Limited quality patient oriented evidence |
Systematic review of lower quality studies or studies with inconsistent findings Low quality clinical trail Cohort studies Case-control studies |
| Level III | Other | Consensus guidelines, extrapolations from bench research, usual practice, opinion, disease-oriented evidence (intermediate or physiological outcomes only), or case series. |
A: Newer product: for most newer products, level I evidence such as high quality systematic reviews or peer-reviewed high quality randomised controlled trials should be identified and referenced in the space provided.
B: Older products: many of these products were developed prior to the wide use of randomised controlled trials. However, there may be level I evidence where the product was used as the control arm for a newer product. If no level 1 evidence can be identified, then level II data from poorer quality controlled trials or high quality observational studies should be referenced in the space provided.
- Cost considerations
Where a published reference supporting the review of cost is available comments should be made regarding its applicability to the South African public sector environment.
Possible unpublished information that can be included:
- Cost per daily dose or course of therapy – for long term or chronic therapy such as hypertension the usual daily dose should be calculated (Dose x number of times a day) and converted into the number of dosing units e.g. tablets. This is then used to calculate the cost per day. For medications used in a course of therapy such as antibiotics this is then multiplied by the number of days in the course of therapy.
- Cost minimisation is used where there is evidence to support equivalence and aims to identify the least costly treatment by identifying all the relevant costs associated with the treatment.
- Cost-effectiveness analysis is used to compare treatment alternatives that differ in the degree of success in terms of the therapeutic or clinical outcome. By calculating a summary measurement of efficiency (a cost-effectiveness ratio), alternatives with different costs, efficacy rates, and safety rates can be fairly compared along a level playing field.
Where any of these have been performed tick the relevant block and send as an attachment with all the calculations. If possible, the spread sheet should be supplied electronically.
Section 3: Motivator’s Details
The receipt of all submissions will be acknowledged. In addition, all decisions with supporting arguments will be communicated where appropriate. This section therefore forms a vital link between the motivator and the decision making process.
1 Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation