T39.1 + (X40.99/X60.99/Y10.99)
DESCRIPTION
Liver damage, due to the depletion of glutathione and accumulation of toxic metabolites, can occur in any individual with paracetamol overdose. Patients with predisposing risk factors for hepatotoxicity (“high risk” patients, see below) may experience toxicity at lower ingested doses.
Clinical features
Gastrointestinal symptoms (anorexia, nausea, vomiting, malaise) predominate in the first 0.5-24 hours. Patients with normal or only slightly raised serum paracetamol levels usually continue to full recovery. In patients with significantly raised paracetamol levels, hepatic toxicity (right upper quadrant abdominal pain and tenderness, elevated bilirubin, raised liver enzymes, coagulation defects, hypoglycaemia, encephalopathy and metabolic acidosis) may manifest from 20-24 hours, peaking in severity at about 72-96 hours. Patients may make a full recovery in 5-7 days, or demise from hepatic failure, or less commonly, renal failure.
"High risk" patients include:
- Chronic alcoholism.
- Chronic liver disease.
- Use of enzyme-inducing medicines (e.g. carbamazepine, phenytoin, efavirenz, phenobarbitone, rifampicin etc.).
- Depletion of glutathione resources (e.g. malnutrition, starvation, AIDS, chronic illness, eating disorders etc.).
- Patients with recent illness, dehydration.
Treatment:
The treatment of paracetamol overdose depends on the dose ingested and the time of presentation since ingestion. A serum paracetamol level is plotted on the nomogram to assess the risk for hepatotoxicity. Values which appear above the treatment line require the antidote N-acetylcysteine (NAC).
Acute single ingestion <8 hours post-ingestion:
Toxic dose defined as >200 mg/kg or 10 g (whichever is less).
Give activated charcoal if the patient presents within 1-2 hours of ingestion
Perform a serum paracetamol level 4 hours post-ingestion
If serum paracetamol level results will not be available before 8 hours post-ingestion, and the patient has taken a toxic dose, do not delay initiation of NAC. It can always be stopped if the serum level plotted on the nomogram does not indicate its continued use.
Acute single ingestion >8 hours post-ingestion:
Toxic dose defined as >200 mg/kg or 10 g (whichever is less).
Start NAC infusion if a toxic dose has been ingested or the patient shows clinical signs of toxicity.
Perform serum paracetamol level, INR and ALT.
Indications for continuing NAC infusion:
- serum paracetamol level above the treatment line on the nomogram
- serum paracetamol level under the treatment line but abnormal ALT
- more than 24 hours post-ingestion, measurable paracetamol level and/or abnormal ALT
Acute single ingestion with unknown time of ingestion:
Manage as for > 8 hours post-ingestion.
Paracetamol treatment nomogram
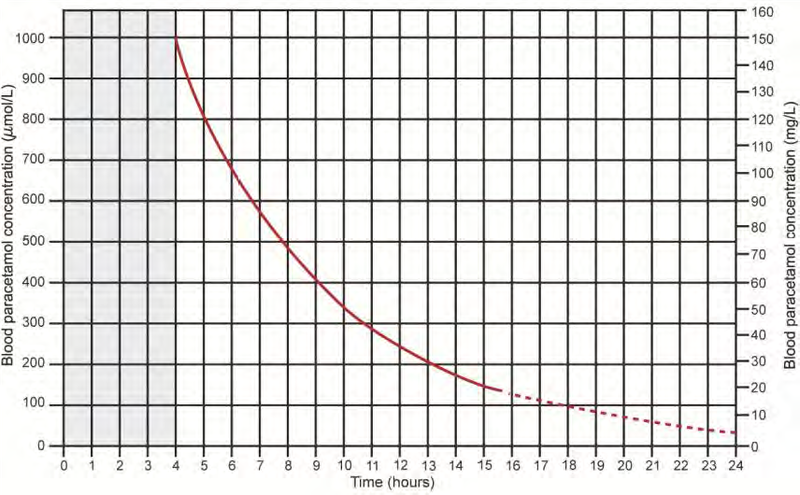
Source: Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA; Panel of Australian and New Zealand clinical toxicologists. Guidelines for the management of paracetamol poisoning in Australia and New Zealand -explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Med J Aust. 2008 Mar 3;188(5):296-301.
(Access the paracetamol nomogram tool on the EML Clinical Guide cellphone application).
Repeated supratherapeutic ingestion (RSTI):
This may occur in patients using repeated high doses of the same product or concurrent use of multiple paracetamol-containing products such as during an acute febrile illness or in patients with chronic pain.
RSTI toxic doses are defined as:
- >200 mg/kg or 10 g (whichever is less) over a single 24-hour period.
- >150 mg/kg or 6 g (whichever is less) per 24-hour period for the preceding 48 hours.
- >100 mg/kg or 4 g/day (whichever is less) per 24-hour period for more than 48 hours and patients have symptoms suggestive of liver injury.
MEDICINE TREATMENT
N-acetylcysteine is the antidote of choice and should be given intravenously. Although it is more effective when given within 8 hours of ingestion of paracetamol, there may be benefit even if liver failure has developed.
Histamine may be released, which mimics an allergic reaction. If this occurs and the patient is stable, infusion may continue at a slower rate under antihistamine cover. In the presence of bronchospasm, stop the infusion.
- N-acetylcysteine, IV:
- Initial infusion: 200 mg/kg in 500 mL 5% dextrose over 4 hours
- Second infusion: 100 mg/kg in 1000 mL 5% dextrose over 16 hours.
- Any further N-acetylcysteine is given according to the second infusion regimen.
If N-acetylcysteine, IV is unavailable:
- N-acetylcysteine, oral, 140 mg/kg, followed by 70 mg/kg 4 hourly for seventeen doses.
Note: Avoid giving activated charcoal if using oral N-acetylcysteine, as it will reduce systemic absorption of the antidote.
Further investigations and referral
Blood tests such as renal function, clotting profile, serum glucose and acid/base status should only be done where clinically indicated.
Patients who develop liver failure must be referred for further management and/or possible transplant.