E78.4/K71.9 + (Y41.5 + B24)
Dyslipidaemia E78.4 + (Y41.5 + B24)
Certain antiretroviral medication, particularly the protease inhibitors, can cause dyslipidaemia. Fasting lipid levels should be done 3 months after starting protease inhibitors. LPV/r is associated with a higher risk of dyslipidaemia (especially hypertriglyceridaemia) than ATV/r.
Patients on LPV/r who:
- develop triglycerides >10 mmol/L;or
- have a total cholesterol >6 mmol/L with a high risk (>20% risk of developing a CVD event in 10 years)
- should switch to ATV/r and repeat the fasting lipid profile in three months.
Patients with persistent dyslipidaemia despite switching to ATV/r, qualify for lipid lowering therapy. Criteria for initiating lipid lowering therapy are the same as for HIV seronegative patients. (See section3.1: Ischaemic heart disease and atherosclerosis, prevention).
Many statins (including simvastatin) cannot be used with protease inhibitors, as protease inhibitors inhibit the metabolism of the statin resulting in extremely high blood levels.
Patients, who fail to respond to lifestyle modification and have hypertriglyceridemia, treat with a fibric acid derivative, e.g.:
- Bezafibrate, oral, 400 mg at night.
OR
If LDL cholesterol is raised (See: Ischaemic heart disease and atherosclerosis, prevention):
- Atorvastatin, oral, 10 mg daily (do not exceed this dose due to a drug interaction with PIs).
Anaemia and neutropenia
AZT causes macrocytosis and can cause anaemia and neutropenia (but note that it does not cause thrombocytopenia). AZT does not need to be stopped with mild anaemia and/or neutropenia, but must be stopped and replaced with an alternative medication if:
- anaemia is symptomatic,
- anaemia is severe (Hb <8.0 g/dL), or
- the neutrophil count is below 0.75 × 109/L.
Lamivudine and emtricitabine can cause pure red cell aplasia, but this is rare.
Hypersensitivity
Note that pre-existing dermatological conditions (especially papulopruritic eruptions and acne) may worsen after commencing ART due to immune reconstitution inflammatory syndrome (see section Management of selected antiretroviral adverse drug reactions) – this is not a hypersensitivity reaction and ART should be continued.
Other medicines, notably cotrimoxazole, can also cause hypersensitivity.
Hypersensitivity rashes occur commonly in the 8-week period after starting NVP or EFV. NNRTI-associated rashes can be severe and life-threatening, especially with nevirapine. If a rash develops on NVP an ALT should be requested urgently.
If any of the following features occur, then NVP or EFV must be permanently discontinued:
- Blistering – if more than 30% of the skin surface is involved this is called Toxic Epidermal Necrolysis, and requires admission.
- Lesions affecting mucous membranes (mouth, eyes, or genitals) – this is called Stevens-Johnson Syndrome, and requires admission
- Fever.
- Features of hepatitis (with nevirapine) – either ALT > 5 times the upper limit of normal or symptomatic hepatitis with deranged liver function tests. Note that the hepatitis usually starts a week or two after the onset of the rash.
With mild rashes NVP and EFV can be continued with careful observation and the rash will often subside. If mild rashes occur on NVP during the dose lead-in phase (200 mg daily) do not increase the dose to 200 mg 12 hourly until the rash improves.
If rash worsens or does not improve within a week discontinue EFV or NVP.
If NVP has been stopped due to cutaneous hypersensitivity, then EFV can be substituted provided that the rash has settled, and that the reaction was not life-threatening (either Stevens-Johnson Syndrome or Toxic Epidermal Necrolysis). If the reaction was life-threatening then a protease inhibitor, e.g. LPV/r, should be substituted.
DTG can cause systemic hypersensitivity syndrome with rash, but this is very uncommon. DTG should be permanently discontinued if this occurs.
ABC can cause a rash as part of a systemic hypersensitivity reaction, which is confined to people who are HLA-B*5701 positive. ABC should be permanently discontinued if this occurs.
Hyperlactataemia
Symptomatic hyperlactataemia occurs due to mitochondrial toxicity of NRTIs. Check for acidosis in such patients.
The estimated risk of lactate elevation differs among the NRTIs, with zidovudine having moderate risk and the other NRTIs low risk.
Risk factors for hyperlactataemia include:
- females,
- obesity,
- prolonged use of NRTIs (> 3 months), or
- development of NRTI-induced peripheral neuropathy or fatty liver.
Clinical symptoms of hyperlactataemia are non-specific and may include:
- nausea
- vomiting
- abdominal pain
- weight loss
- malaise
- tachycardia
- liver dysfunction (due to steatosis)
A high index of suspicion is necessary. Send blood for lactate levels (check with your local laboratory for specimen requirements for lactate). Alternatively, point of care finger prick lactate monitoring can be done. Check the serum bicarbonate level.
Patients with mild hyperlactataemia (lactate 2.5–5 mmol/L):
Therapy should be altered by selecting NRTIs that are less associated with hyperlactataemia.
Monitor serial lactate measurements until the lactate has returned to within the normal range.
Note: The resolution of hyperlactataemia may take a few months.
Patients with lactate levels > 5 mmol/L:
Stop the NRTIs.
If the patient is on a 1st line regimen, continue the EFV or DTG and add LPV/r.
If the patient is on the 2nd line regimen, consult with an HIV specialist.
If there is acidosis, then admission to a high care unit is recommended.
Lactic acidosis carries a poor prognosis. Treatment is largely supportive. It is essential to exclude other causes of lactic acidosis, especially sepsis. High dose vitamin B, especially riboflavin and thiamine, may have a role in therapy.
Hepatotoxicity K71.9 + (Y41.5 + B24)
All currently available antiretrovirals are potentially hepatotoxic. EFV has the highest risk. NRTIs uncommonly cause acute hepatitis, but may result in steatohepatitis after prolonged use, which manifests with mildly elevated liver enzymes, affecting GGT and alkaline phosphatase more than the transaminases, and ALT more than AST. Patients on atazanavir may develop jaundice due an unconjugated hyperbilirubinaemia, which is not accompanied by liver injury. This is a cosmetic issue and the atazanavir can be substituted if the patient is unable to tolerate the jaundice. However, all protease inhibitors can rarely cause hepatitis, so it is important to exclude this in patients developing jaundice on ATV/r.
Other potentially hepatotoxic medicines prescribed to in HIV-infected patients include anti-tuberculous therapy, fluconazole and cotrimoxazole. Cotrimoxazole, amoxicllin/clavulanate and macrolides tend to cause cholestatic hepatitis that may take months to resolve.
The exclusion of viral hepatitis is important in the work-up of drug-induced liver injury (DILI). Testing for hepatitis A, B and C should be undertaken. Hepatitis B is common, and flares of viral hepatitis may occur after ART initiation. Furthermore, life threatening flares may occur when antiretrovirals that are also active against hepatitis B (TDF, 3TC and FTC) are withdrawn.
Other potential causes include disseminated TB, IRIS, alcohol, alternative remedies, fatty liver, sepsis and HIV cholangiopathy.
Investigations:
- Request an ALT.
- Request viral hepatitis screen, full liver function tests and INR in patients if ALT >5 x upper limit of normal (ULN) and/or jaundice and/or symptoms of hepatitis are present.
- Perform a liver ultrasound if GGT or ALP are significantly elevated or if conjugated bilirubin is elevated, to exclude:
- Extrahepatic biliary obstruction.
- Fatty liver due to NRTIs.
- Disseminated TB.
| Upper Limit of Normal (ULN) | <2.5 x ULN | 2.5 – 5 x ULN | > 5 x ULN |
| ALT | Repeat in 2 weeks | Repeat in 1 week | Stop ART |
| Isolated Hyperbilirubinaemia | Repeat in 1 week | Stop ART | Stop ART |
*Stop the relevant medicines at lower levels if symptoms of hepatitis (right upper quadrant pain, nausea / vomiting) or jaundice are present.
If ART is considered to be the cause substitute ART as follows:
- If the hepatitis occurred on efavirenz, substitute with DTG or a boosted PI.
- If hepatitis occurred on PI, substitute with DTG.
- NRTI fatty liver – discontinue AZT (if relevant) and replace with safer NRTI (TDF or ABC) – if not on AZT and hepatitis is severe switch to NRTI-sparing regimen.
Hepatitis in patients on ART and anti-tuberculosis therapy
Drug-induced liver injury (DILI) is a known adverse effect of anti-tuberculosis therapy and ART and is a common problem in HIV/TB co-infected patients. First-line TB medicines associated with DILI include isoniazid (INH), rifampicin (RIF) and pyrazinamide (PZA). Anti-tuberculosis therapy commonly causes transient, mild, asymptomatic elevations in serum aminotransferase levels not requiring discontinuation of therapy.
If hepatitis develops, as defined above, stop all antiretrovirals, cotrimoxazole and all potentially hepatotoxic TB medicines (isoniazid, rifampicin and pyrazinamide).
TB immune reconstitution inflammatory syndrome (TB-IRIS) should be considered in the differential diagnosis (see: Management of selected antiretroviral adverse drug reactions). This condition presents shortly after ART initiation in patients with TB. The GGT and ALP are elevated to a greater degree than the transaminases. Mild jaundice with a conjugated hyperbilirubinaemia and tender hepatosplenomegaly may be present.
Investigations:
- Request an ALT.
- Request viral hepatitis screen, full liver function tests and INR in patients if ALT >5 x ULN and/or jaundice and/or symptoms of hepatitis are present.
- Perform a liver ultrasound if GGT or ALP are significantly elevated or if conjugated bilirubin is elevated, to exclude extrahepatic biliary obstruction.
- Reassess the grounds for TB diagnosis.
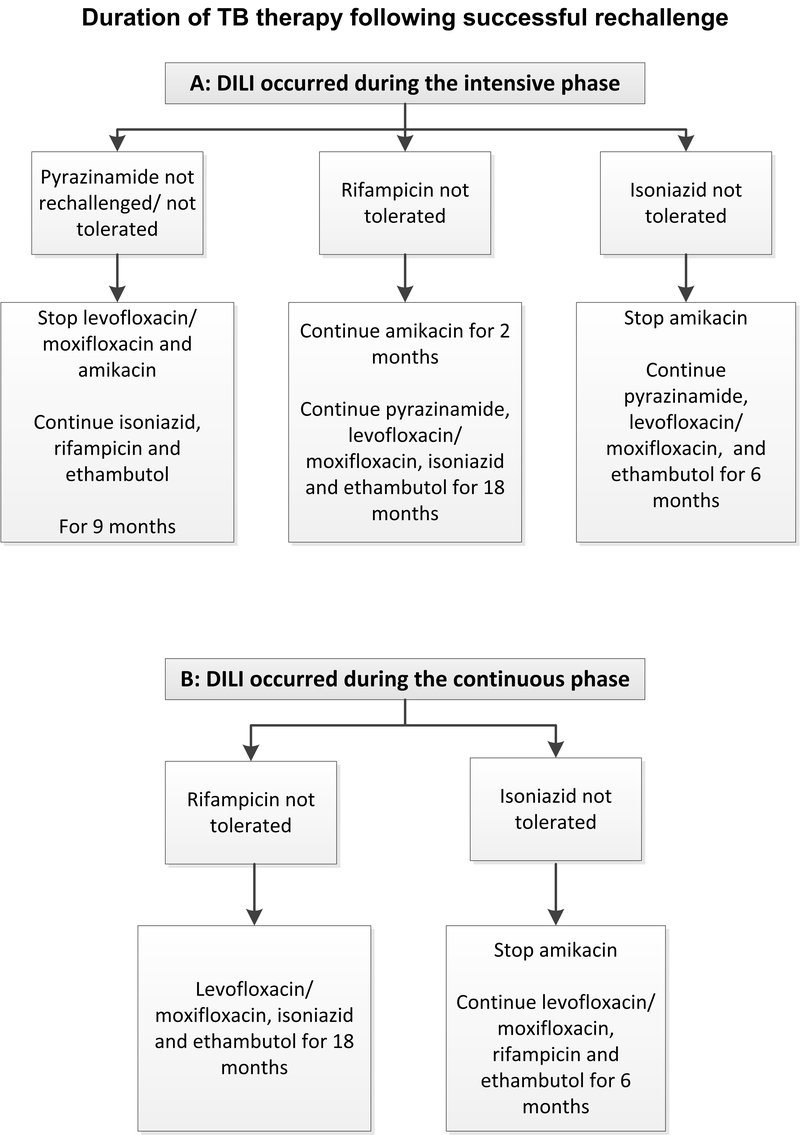
- Check if patient is on intensive or continuation phase of TB treatment.
Management:
- Stop TB therapy and initiate background TB therapy and continue throughout rechallenge:
- Amikacin, IV, 15 mg/kg daily.
- Moxifloxacin, oral, 400 mg daily or levofloxacin 750–1000 mg daily.
- Ethambutol, oral, 800–1200 mg daily.
- Stop cotrimoxazole prophylaxis and do not rechallenge.
- Stop ART as described above.
- Repeat ALT and bilirubin in 2 days (inpatient) or 7 days (outpatient).
- When ALT is <100 IU/L and total bilirubin is less than twice the upper limit of normal, start TB medicine rechallenge as follows:
| Day 1: |
· Rifampicin, oral 600 mg daily. If <60 kg: rifampicin, oral 450 mg daily. |
|---|---|
| Day 3: | » Check ALT. |
| Day 4–6: |
ADD · Isoniazid, oral 300 mg daily. |
| Day 7: | » Check ALT. |
| Day 8: |
» Stop moxifloxacin/levofloxacin and amikacin. Consider a pyrazinamide rechallenge (in cases of TB meningitis or intolerance/resistance to other medicines). · Pyrazinamide, oral 25 mg/kg daily. |
| Day 10: |
» Check ALT. » Thereafter, monitor ALT twice weekly for the first 3 weeks, then every two weeks for a month, then monthly until 3 months. · Restart ART 2 weeks after completing rechallenge of TB therapy. Monitor ALT every 2 weeks for 2 months after ART rechallenge. |